Lab overview
In order to keep us healthy, the immune system must recognize and destroy a wide range of invading pathogens, while limiting damage to healthy tissues. This is accomplished by a complex network of immune cells that continuously survey the body in order to rapidly respond to pathogenic insults.
My lab is focused on understanding how cell-to-cell communicative behavior of immune cells is regulated within healthy tissue, and how these behaviors are altered in response to an infection in vivo.
We use multiphoton intravital microscopy (MP-IVM) to visualize the migration, dynamic behavior and localization of immune cells within their physiological tissue in live, anaesthetized mice. Our dedicated imaging facility allows us to perform MP-IVM in infected animals that require BSL2+ containment. The goal is to use microscopy and analytical approaches to characterize the relationship between cellular motility, intercellular communication and immunity using novel imaging techniques.
In order to keep us healthy, the immune system must recognize and destroy a wide range of invading pathogens, while limiting damage to healthy tissues. This is accomplished by a complex network of immune cells that continuously survey the body in order to rapidly respond to pathogenic insults.
My lab is focused on understanding how cell-to-cell communicative behavior of immune cells is regulated within healthy tissue, and how these behaviors are altered in response to an infection in vivo.
We use multiphoton intravital microscopy (MP-IVM) to visualize the migration, dynamic behavior and localization of immune cells within their physiological tissue in live, anaesthetized mice. Our dedicated imaging facility allows us to perform MP-IVM in infected animals that require BSL2+ containment. The goal is to use microscopy and analytical approaches to characterize the relationship between cellular motility, intercellular communication and immunity using novel imaging techniques.
Current research projects
|
|
Dynamics of macrophage:T cell interactions during HIV infection and their role in viral spread.
HIV-infection of macrophages induces prolonged interactions with motile T cells, thereby increasing virus transmission. Macrophage-to-T cell viral spread is less sensitive to single antiretroviral drug therapy and involves both gp120:CD4 and LFA-1:ICAM-1 interactions. Lopez et al, Journal of Virology; 2019. |
|
Visualizing anti-Leishmanial CD4 T cell responses during acute infection and healed skin in vivo.
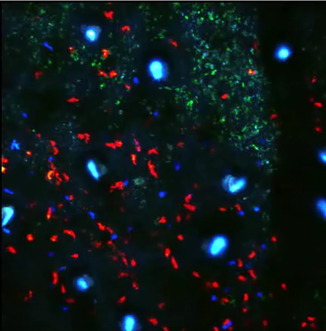
Leishmania major is an intracellular parasite that has evolved several strategies to evade the immune system. While strong T cell responses are generated during acute infection, Leishmania-infected cells persist indefinitely even after skin healing and can reactivate to cause pathology. Thus, a gap in knowledge is why T cells fail to completely remove infected cells, and the cellular mechanisms that are in place that supports parasite persistence. We are using MP-IVM to visually characterize Leishmania-specific T cell responses during acute and persistent infection to better describe the interplay between T cell motility, effector function and cellular distribution. |
Adoptive transfer of Leishmania-specific (blue) or control (red) T cells into Leishmania major infected lesions in the ear skin (green).
|
|
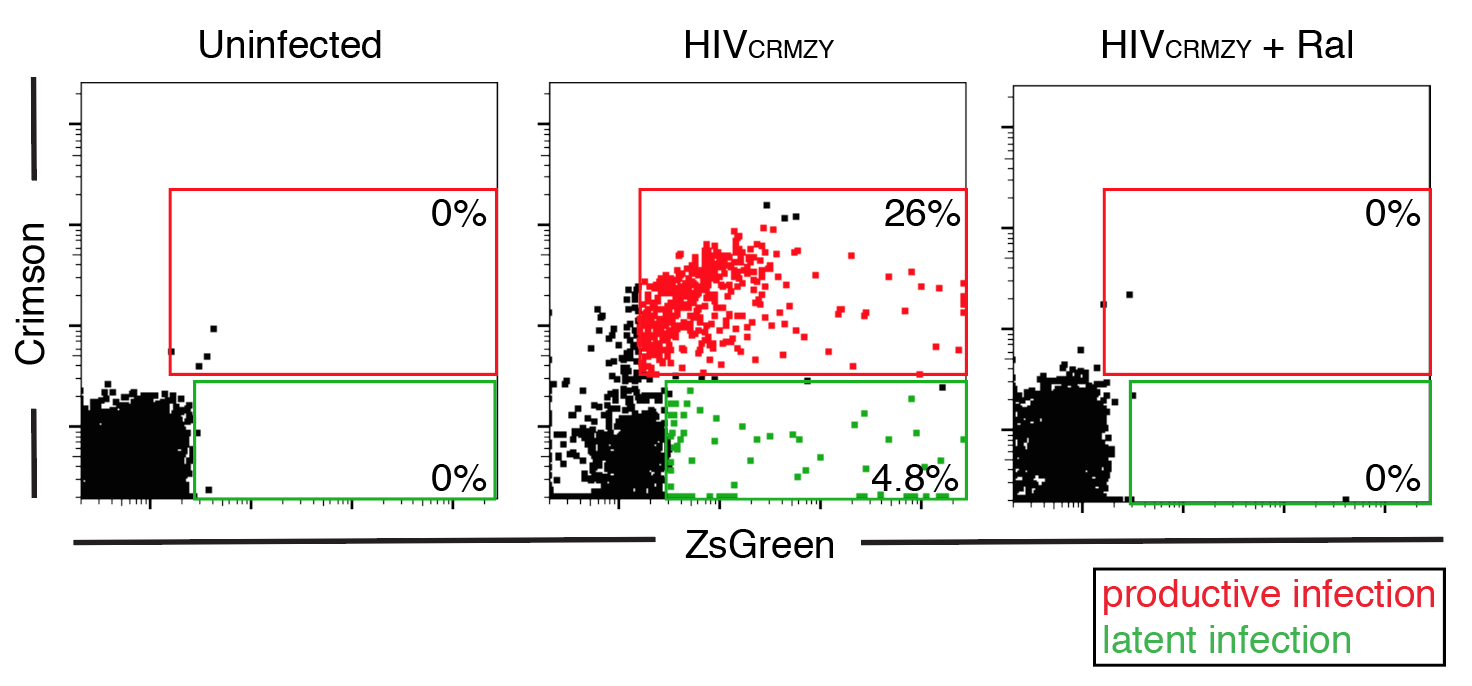
CD4 T cells were infected with a dual-fluorescent reporter (HIV-CRMZY) to discriminate between productive (red) and latent (green) infection.
|
Modeling HIV latency generation and maintenance in T cells.
HIV can remain dormant, or latent, in infected T cells for many years, and represents a major barrier to HIV cure. Previous studies have demonstrated that myeloid cells can enhance latency in T cells, suggesting that activation of specific signals during cell-to-cell contact may induce latency during infection. We have constructed a new dual-reporter HIV reporter that will allow us to visually discriminate productively- from latently-infected cells, to test which signals are the drivers of latency generation and maintenance. |
|
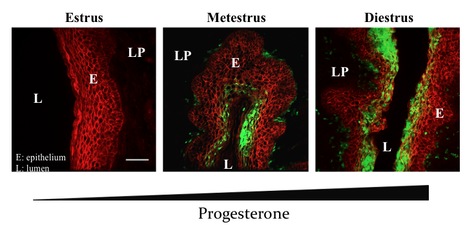
Role of mucosal neutrophils on vaginal epithelial barrier function and their interplay with the vaginal microbiome.
Women with a polymicrobial vaginal flora exhibit higher levels of inflammation, epithelial barrier dysfunction and elevated heterosexual HIV risk. Increased presence of neutrophils and neutrophil-derived factors also strongly correlate with higher HIV risk, suggesting that vaginal neutrophils may enhance HIV susceptibility. However, no direct demonstration of how mucosal neutrophils are able to remodel the vaginal mucosal landscape in response to different microbes is lacking. We have developed mouse models to study the interplay between neutrophils and vaginal microbes in vivo in order to place a biological function to neutrophils during HIV infection. |
Massive accumulation of Ly6G+ neutrophils (green) into the vaginal epithelium (E; red) and lamina propria (LP) due to progresterone secretion. L = lumen.
|
Movie gallery
|
Large "dendritic-like" syncytium in the lymph node.
|
|
|
Intravital imaging of a large syncytium at day 6 post-infection in the lymph node of a humanized BLT mouse. Mice were infected with an CCR5-tropic HIV-GFP reporter by footpad injection and the draining popliteal lymph node prepared for MP-IVM Murooka et al, AIDS and Human Retroviruses; 2015. |
|
HIV-1 infection induces an "elongated" phenotype
in some lymph node cells. |
|
|
MP-IVM of HIV-infected cells with various morphologies and behaviors at day 2 post-infection in the lymph node of a humanized BLT mouse. Elongated phenotypes were highly dynamic, and some reached over 100 microns in length. Murooka et al, Nature; 2012. |
|
HIV-1 infection of macrophages form elongated "tethers" with migrating T cells.
|
|
|
|
|
Motile DCs capture and compartmentalize HIV particles
|
|
Live-cell imaging of motile DCs exposed to GFP-labeled HIV particles. DCs immediately capture and compartmentalize viral particles in the rear-end of migrating cells that can facilitate the transfer of viral particles to T cells through transient DC:T cell interactions. Koh et al, iScience; 2020. |